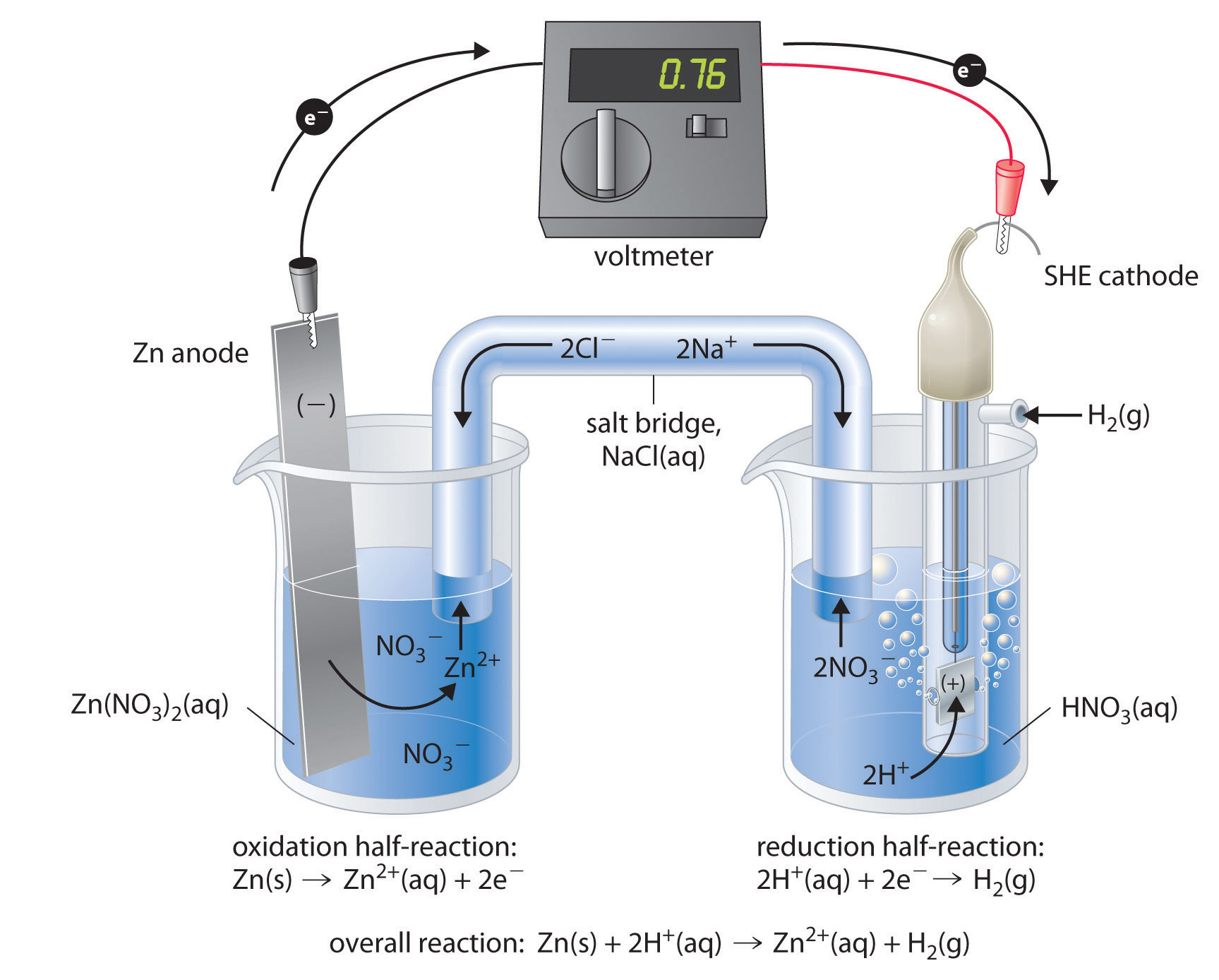
Hydrogen Electrode Is Dipped In A Solution . it is essentially a platinum electrode coated with platinum black and submerged in a solution with a hydrogen ion activity of 1. The negative electrode is in contact with a solution of ph = 6. The correct option is d −0.177 v. The reduction potential of the electrode would be (the. We know, ered =e0 red +0.059 log[h+] ⇒ e. for example, when a hydrogen electrode is dipped in an acidic solution with ph 3 at 25 degrees celsius, we can determine its. a cell is containing two h electrodes. standard hydrogen electrode construction. the hydrogen electrode is dipped in a solution of p h = 3 at 25 ∘ c. The structure of the standard hydrogen. The parts that make up a standard hydrogen electrode are listed below.
from saylordotorg.github.io
the hydrogen electrode is dipped in a solution of p h = 3 at 25 ∘ c. The reduction potential of the electrode would be (the. The structure of the standard hydrogen. for example, when a hydrogen electrode is dipped in an acidic solution with ph 3 at 25 degrees celsius, we can determine its. The parts that make up a standard hydrogen electrode are listed below. it is essentially a platinum electrode coated with platinum black and submerged in a solution with a hydrogen ion activity of 1. The correct option is d −0.177 v. We know, ered =e0 red +0.059 log[h+] ⇒ e. The negative electrode is in contact with a solution of ph = 6. standard hydrogen electrode construction.
Electrochemistry
Hydrogen Electrode Is Dipped In A Solution The negative electrode is in contact with a solution of ph = 6. standard hydrogen electrode construction. it is essentially a platinum electrode coated with platinum black and submerged in a solution with a hydrogen ion activity of 1. We know, ered =e0 red +0.059 log[h+] ⇒ e. for example, when a hydrogen electrode is dipped in an acidic solution with ph 3 at 25 degrees celsius, we can determine its. the hydrogen electrode is dipped in a solution of p h = 3 at 25 ∘ c. The structure of the standard hydrogen. The parts that make up a standard hydrogen electrode are listed below. The reduction potential of the electrode would be (the. The negative electrode is in contact with a solution of ph = 6. The correct option is d −0.177 v. a cell is containing two h electrodes.
From www.toppr.com
The hydrogen electrode is dipped in a solution of pH = 3 at 25 ^o C Hydrogen Electrode Is Dipped In A Solution The negative electrode is in contact with a solution of ph = 6. standard hydrogen electrode construction. We know, ered =e0 red +0.059 log[h+] ⇒ e. the hydrogen electrode is dipped in a solution of p h = 3 at 25 ∘ c. The parts that make up a standard hydrogen electrode are listed below. The reduction potential. Hydrogen Electrode Is Dipped In A Solution.
From www.doubtnut.com
If hydrogen electrodes dipped in two solutions of pH=3 and pH=6 are co Hydrogen Electrode Is Dipped In A Solution The parts that make up a standard hydrogen electrode are listed below. The structure of the standard hydrogen. The correct option is d −0.177 v. it is essentially a platinum electrode coated with platinum black and submerged in a solution with a hydrogen ion activity of 1. the hydrogen electrode is dipped in a solution of p h. Hydrogen Electrode Is Dipped In A Solution.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts Hydrogen Electrode Is Dipped In A Solution The structure of the standard hydrogen. The negative electrode is in contact with a solution of ph = 6. for example, when a hydrogen electrode is dipped in an acidic solution with ph 3 at 25 degrees celsius, we can determine its. The parts that make up a standard hydrogen electrode are listed below. it is essentially a. Hydrogen Electrode Is Dipped In A Solution.
From www.toppr.com
The hydrogen electrode is dipped in a solution of pH = 3 at 25 ^o C Hydrogen Electrode Is Dipped In A Solution The parts that make up a standard hydrogen electrode are listed below. it is essentially a platinum electrode coated with platinum black and submerged in a solution with a hydrogen ion activity of 1. The reduction potential of the electrode would be (the. a cell is containing two h electrodes. for example, when a hydrogen electrode is. Hydrogen Electrode Is Dipped In A Solution.
From stock.adobe.com
Standard hydrogen electrode diagram. Scientific vector illustration Hydrogen Electrode Is Dipped In A Solution a cell is containing two h electrodes. The negative electrode is in contact with a solution of ph = 6. standard hydrogen electrode construction. The correct option is d −0.177 v. We know, ered =e0 red +0.059 log[h+] ⇒ e. the hydrogen electrode is dipped in a solution of p h = 3 at 25 ∘ c.. Hydrogen Electrode Is Dipped In A Solution.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Hydrogen Electrode Is Dipped In A Solution a cell is containing two h electrodes. standard hydrogen electrode construction. the hydrogen electrode is dipped in a solution of p h = 3 at 25 ∘ c. The correct option is d −0.177 v. The structure of the standard hydrogen. The reduction potential of the electrode would be (the. We know, ered =e0 red +0.059 log[h+]. Hydrogen Electrode Is Dipped In A Solution.
From monomole.com
Standard hydrogen electrode Mono Mole Hydrogen Electrode Is Dipped In A Solution the hydrogen electrode is dipped in a solution of p h = 3 at 25 ∘ c. standard hydrogen electrode construction. We know, ered =e0 red +0.059 log[h+] ⇒ e. The structure of the standard hydrogen. The correct option is d −0.177 v. The parts that make up a standard hydrogen electrode are listed below. it is. Hydrogen Electrode Is Dipped In A Solution.
From askfilo.com
EC0067 55. The hydrogen electrode is dipped in a solution of pH=3 at 25∘C.. Hydrogen Electrode Is Dipped In A Solution The structure of the standard hydrogen. The correct option is d −0.177 v. The reduction potential of the electrode would be (the. a cell is containing two h electrodes. The negative electrode is in contact with a solution of ph = 6. The parts that make up a standard hydrogen electrode are listed below. it is essentially a. Hydrogen Electrode Is Dipped In A Solution.
From saylordotorg.github.io
Electrochemistry Hydrogen Electrode Is Dipped In A Solution We know, ered =e0 red +0.059 log[h+] ⇒ e. The parts that make up a standard hydrogen electrode are listed below. a cell is containing two h electrodes. for example, when a hydrogen electrode is dipped in an acidic solution with ph 3 at 25 degrees celsius, we can determine its. The correct option is d −0.177 v.. Hydrogen Electrode Is Dipped In A Solution.
From byjus.com
What is standard hydrogen electrode? Hydrogen Electrode Is Dipped In A Solution The structure of the standard hydrogen. We know, ered =e0 red +0.059 log[h+] ⇒ e. a cell is containing two h electrodes. The reduction potential of the electrode would be (the. The correct option is d −0.177 v. standard hydrogen electrode construction. The parts that make up a standard hydrogen electrode are listed below. it is essentially. Hydrogen Electrode Is Dipped In A Solution.
From www.toppr.com
The hydrogen electrode is dipped in a solution of pH = 3 at 25 ^o C Hydrogen Electrode Is Dipped In A Solution the hydrogen electrode is dipped in a solution of p h = 3 at 25 ∘ c. standard hydrogen electrode construction. The parts that make up a standard hydrogen electrode are listed below. it is essentially a platinum electrode coated with platinum black and submerged in a solution with a hydrogen ion activity of 1. a. Hydrogen Electrode Is Dipped In A Solution.
From www.toppr.com
The hydrogen electrode is dipped in a solution of pH = 3 at 25 ^o C Hydrogen Electrode Is Dipped In A Solution a cell is containing two h electrodes. The correct option is d −0.177 v. The structure of the standard hydrogen. We know, ered =e0 red +0.059 log[h+] ⇒ e. it is essentially a platinum electrode coated with platinum black and submerged in a solution with a hydrogen ion activity of 1. standard hydrogen electrode construction. for. Hydrogen Electrode Is Dipped In A Solution.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Hydrogen Electrode Is Dipped In A Solution The correct option is d −0.177 v. We know, ered =e0 red +0.059 log[h+] ⇒ e. for example, when a hydrogen electrode is dipped in an acidic solution with ph 3 at 25 degrees celsius, we can determine its. the hydrogen electrode is dipped in a solution of p h = 3 at 25 ∘ c. it. Hydrogen Electrode Is Dipped In A Solution.
From www.toppr.com
The hydrogen electrode is dipped in a solution of pH = 3 at 25^oC . The Hydrogen Electrode Is Dipped In A Solution We know, ered =e0 red +0.059 log[h+] ⇒ e. it is essentially a platinum electrode coated with platinum black and submerged in a solution with a hydrogen ion activity of 1. The negative electrode is in contact with a solution of ph = 6. standard hydrogen electrode construction. The structure of the standard hydrogen. the hydrogen electrode. Hydrogen Electrode Is Dipped In A Solution.
From www.numerade.com
SOLVED A hydrogen electrode is immersed in a 0.10 M solution of acetic Hydrogen Electrode Is Dipped In A Solution The reduction potential of the electrode would be (the. the hydrogen electrode is dipped in a solution of p h = 3 at 25 ∘ c. standard hydrogen electrode construction. it is essentially a platinum electrode coated with platinum black and submerged in a solution with a hydrogen ion activity of 1. The structure of the standard. Hydrogen Electrode Is Dipped In A Solution.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID752723 Hydrogen Electrode Is Dipped In A Solution for example, when a hydrogen electrode is dipped in an acidic solution with ph 3 at 25 degrees celsius, we can determine its. The parts that make up a standard hydrogen electrode are listed below. the hydrogen electrode is dipped in a solution of p h = 3 at 25 ∘ c. it is essentially a platinum. Hydrogen Electrode Is Dipped In A Solution.
From www.toppr.com
The hydrogen electrode is dipped in a solution of pH = 3 at 25^oC The Hydrogen Electrode Is Dipped In A Solution The structure of the standard hydrogen. The reduction potential of the electrode would be (the. The correct option is d −0.177 v. The negative electrode is in contact with a solution of ph = 6. the hydrogen electrode is dipped in a solution of p h = 3 at 25 ∘ c. it is essentially a platinum electrode. Hydrogen Electrode Is Dipped In A Solution.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Hydrogen Electrode Is Dipped In A Solution The reduction potential of the electrode would be (the. the hydrogen electrode is dipped in a solution of p h = 3 at 25 ∘ c. it is essentially a platinum electrode coated with platinum black and submerged in a solution with a hydrogen ion activity of 1. The correct option is d −0.177 v. The negative electrode. Hydrogen Electrode Is Dipped In A Solution.